Today, Canada’s healthcare industry is experiencing great demand from the advance of chronic disease to an aging population and everything in between. In turn, the demand on the healthcare industry has increased the need for high-quality medical devices. Medical devices encompass a broad range of products from first aid kits to crutches all the way to pacemakers.
According to the International Trade Administration, medical device imports account for nearly 75% of the medical device market. The United States is the leading supplier of medical devices to many countries including Canada. To meet demand, successful cross-border shipping is essential.
Medical devices are highly regulated federally by Health Canada’s Medical Devices Bureau of the Therapeutic Products Directorate (TPD) and governed by Canada’s Food and Drugs Act (F&DA) and Canadian Medical Device Regulations (CMDR).
Due to strict standards, shipping medical devices into Canada can be a challenging task. To help you quickly and reliably deliver medical equipment, we’ve created a resource that tackles the nuances of cross-border shipping.
Know your medical device classification
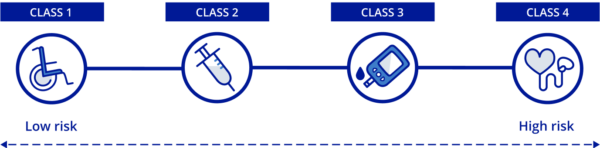
There are four medical device classifications that are ranked by risk factor. The risk factors determine the regulations the medical device will be held accountable to for importation. Therefore, the higher the classification, the higher the risk factor and stricter the importing criteria.
The four medical device classes are:
- Class 1 (lowest risk): examples include non-electric wheelchairs and surgical tools
- Class 2 (low risk): examples include syringes and catheters
- Class 3 (moderate risk): examples include glucose monitors and orthopedic implants
- Class 4 (high risk): examples include pacemakers and mechanical heart valves
For all medical device classes, the importer must have a valid Medical Device Establishment License (MDEL). For class 2, 3 and 4 medical devices, the device must have a Medical Device License (MDL).
Complete your labels
To import medical devices, there must be a clearly placed label on the outside of the package that includes:
- The product’s name
- Name of the business and location
- The product’s identity (e.g. type of medical device )
- Class 3 and 4 medical devices must have a control number on the label
- The components within the box (e.g. weight and size)
- The word “sterile” printed if the medical device will be sold
- The expiration date, if needed
- The product’s directions and potential dangers
As a requirement of the Canadian Consumer Packaging and Labeling Act, medical devices being sold for individual use need to have directions printed in English and French.
For information on the best way to package your healthcare shipments, check out our comprehensive infographic.
Identify your Incoterms
Incoterms are 11 internationally recognized rules that define the responsibilities of sellers and buyers in international transactions. Issued by the International Chamber of Commerce (ICC), these rules cover responsibilities such as who is to pay for and manage the shipment, insurance and customs clearance.
Familiarizing yourself with Incoterms helps create smoother processes by clearly defending which party is responsible at each step of the shipment.
The Incoterms are grouped into two categories which reflect modes of transport.
Firstly, there are seven rules for any mode of transport:
- EXW – Ex Works (insert place of delivery)
- FCA – Free Carrier (insert named place of delivery)
- CPT – Carriage Paid to (insert place of destination)
- CIP – Carriage and Insurance Paid to (insert place of destination)
- DAP – Delivered at Place (insert named place of destination)
- DPU – Delivered at Place Unloaded (insert of place of destination)
- DDP – Delivered Duty Paid (insert place of destination).
Next, there are four rules for sea and inland and waterway transport:
- FAS – Free Alongside Ship (insert name of port of loading)
- FOB – Free on Board (insert named port of loading)
- CFR – Cost and Freight (insert named port of destination)
- CIF – Cost Insurance and Freight (insert named port of destination)
Avoiding delays
Even the most meticulous teams can run into delays when it comes to medical equipment shipping. To avoid delays, keep on top of the changing regulatory environment. Also, ensure that you prepare your paperwork early and submit it correctly. Lastly, identify opportunities to consolidate shipments for efficiency.
 An expedited cross-border case study
An expedited cross-border case study
Medical device shippers can learn from other vertical markets with similar pain points about crossing the border. Global power tool and floor care manufacturer TTI was dissatisfied with their previous delivery partners that hired third parties to complete Canadian deliveries. This created a fragmented and bottlenecked shipping process that was limiting TTI’s growth. To streamline deliveries, TTI contacted Purolator. Together, Purolator and TTI provided customers with full tracking visibility and considerably faster shipping. Janice Amenta, Director of Operations for the Milwaukee division of TTI, said, “Transit times have gone from around 14 days down to – in some areas – four or five business days.”
What to do if items are refused at the border
Whether it’s incorrect Incoterms or bond issues, there are various reasons why a shipment can be stopped at the border. Whatever the issue may be, the border will notify the shipper of the reason. It’s essential to work with a courier that has a strong relationship with the border in order to keep your shipments moving. By tapping into their team of trade compliance specialists, your provider should strive to make each shipment streamlined and stress-free at every step.
What solutions should a delivery provider offer for cross-border shipping?
To ensure your medical devices arrive at their destination safely, quickly and in good condition while crossing borders, you need a trusted logistics partner with experience in cross-border shipping. Look for a delivery provider that can offer the following solutions:
- Cross-border expertise and support. To guarantee you’re compliant and understand the process of cross-border shipping.
- Large network within Canada. Ensure that you can deliver across Canada, even to the most remote regions.
- U.S. and Canada locations. Secure seamless shipment border crossing with a provider that has representation in the U.S and Canada.
- Customized solutions. Create a personalized solution tailored to your business’ unique needs.
- An array of delivery time frames. From 2-day express to Mission Critical, select from a variety of time frames to accommodate each delivery.
- No handoffs. Your shipment remains in the hands of one delivery provider throughout its shipping journey, decreasing the chance of theft and damage.
- Full visibility. From the medical device’s manufacturer to its final destination, track shipments 24/7 with real-time updates.
How to organize shipping cross-border repairs
For many medical equipment shipments, time is of the utmost essence. To meet existing service-level agreements, quickly delivering repair parts to repair technicians is crucial. Leveraging an expedited shipping method, such as Purolator’s Quickship service, will guarantee that you can ship next day, seven days a week and fulfill more orders with extended hour pickups and deliveries. By managing quick and reliable medical equipment repair part shipments, you’ll also be extending the life of the medical equipment.
How to manage returns
Return requests happen. Being prepared for them is just as important as the initial shipment. To manage returns efficiently, you need a logistics partner that can help minimize the impact on your bottom line and even help you with promoting a circular economy. Your cross-border shipping partner should:
- Cover return merchandise authorization (RMA), including returning items to the supplier
- Manage warranties
- Recycle salvageable components or offer safe disposal in compliance with regulations
By following our cross-border medical equipment shipping advice, you are one step closer to guaranteeing safe, swift and reliable deliveries. Delivering medical devices across the border doesn’t need to feel challenging. Partner with a trusted logistics provider like Purolator to make the process seamless and secure. This way, you can always deliver urgently needed medical devices without complications.


 An expedited cross-border case study
An expedited cross-border case study